PhD Studentships
Research groups
We currently have three funded studentships available.1. Mathematical modelling of macromolecular capillary permeability
Supervisors: Dr
Kenton Arkill (Medicine), Dr
Reuben O’Dea (Maths), Professor
David Bates (Medicine), Dr
Matthew Hubbard (Maths)
The primary function of blood vessels is to transport molecules to tissues. In diseases such as cancer and diabetes this transport, particularly of large molecules such as albumin, can be an order of magnitude higher than normal.
The project is to model transient flow of macromolecules across the vascular wall in physiology and pathology. The doctoral student will join a team that includes medical researchers, biophysicists and mathematicians acquiring structural and functional data.
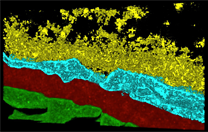
Detailed microscale models of vascular wall
hydrodynamics and transport properties will be
employed; in addition, powerful multiscale
homogenisation techniques will be exploited that
enable permeability and convection parameters on the
nanoscale to be linked through the microscale into
translatable information on the tissue scale.
Computational simulations will be used to investigate
and understand the model behaviour, including, for
example, stochastic and multiphysics effects in the
complex diffusion-convection nanoscale environment.
The project will afford a great opportunity to form an
information triangle where modelling outcomes will
determine physiological experiments to feedback to the
model. Furthermore, the primary results will inform
medical researchers on potential molecular therapeutic
targets.
apply online via the University of Nottingham application page
3. Modelling the alternative splicing of tissue growth regulators and its implications for tumour growth
Supervisors: Professor Markus Owen (School of Mathematical Sciences), Professor David Bates (Division of Cancer and Stem Cells, School of Medicine)
Normal and pathological tissue growth is regulated by diverse growth factors and related molecules, many of which are produced in cells via the transcription of associated genes and translation of mRNA to protein. In many cases, alternative splicing, regulated by splicing factors, leads to different isoforms of proteins, which can have different effects. This is particularly pertinent to angiogenesis, the process whereby new blood vessels are produced from existing ones, which is crucial in cancer and also diseases such as diabetic retinopathy.
Different isoforms of Vascular Endothelial Growth Factor (VEGF), whose balance is regulated by alternative splicing, can promote or inhibit angiogenesis. In fact, the relevant splicing factors seem to regulate alternative splicing of families of genes controlling cell death, growth factor signaling, the cell cycle, invasion and immune responses. Thus it important to consider the overall effect of splicing factors in the context of a whole tissue where all these processes are modulated.
This project will focus on mathematical modelling of the various aspects of alternative growth factor splicing, regulation of angiogenesis, and tumour growth, with the following objectives:
- model splicing control at network level;
- model the implications for tissue growth of altered splicing control
- couple O1 and O2 to predict the efficacy of interventions that modulate alternative splicing in cancer.
This will require the develop and application of advanced mathematical and computational techniques to make the link from molecules to cells to tissues. A significant challenge is to use a blend of mathematical and statistical approaches to allow the translation of varied experimental data and knowledge into tractable parameterised mathematical frameworks that combine dynamics over a range of scales.
This project would also involve co-operation with
Exonate, a biopharmaceutical company focussed on the
discovery and development of small molecule drugs
that modulate alternative mRNA splicing to address
diseases of high unmet medical need. Exonate will
provide relevant data and scientific input, and also
contribute to the student training, for example by
through hosting them within the company on
secondment.
apply online via the University of Nottingham application page
References
- M R Owen et al. Cancer Res 71(8) 2826-37 (2011)
2. Investigating the neurovascular unit in diabetic neuropathic pain
based at Nottingham Trent University with Dr Richard Hulse as the primary supervisor.
A
fully funded 3 year PhD studentship is available
within the School of Science and Technology at
Nottingham Trent University, supported by
EFSD/Boehringer Ingelheim European Research Programme
in Microvascular Complications of Diabetes. The
project is based in research laboratories specialising
in pain (Dr Richard Hulse) and diabetes (Prof Philip
McTernan), in collaboration with the Tumour and
Vascular Biology Laboratories, University of
Nottingham (Prof David Bates).
Neuropathic pain is a
burden many people with diabetes suffer from. Despite
this extensive clinical need for effective analgesic
treatment, many current painkilling treatments are
ineffective and/or have significant adverse
side-effects. Our research focuses upon understanding
the diabetes induced pathology surrounding sensory
neuronal degeneration and pain. Diabetic
patients are highly susceptible to microvascular
disease, which is associated with neurodegenerative
disease in these patients. Our recent work demonstrates that
diabetes damages the blood vessels in the sensory
nervous system (Ved et al. 2018. J. Physiol). Your
research project will find out how these
neuro-vascular interactions can alter pain processing.
Identifying how blood vessels and sensory neurons
communicate will allow you to find out how alterations
in these pathways contribute to the development of
diabetic neuropathy, opening up new treatment options
for people with diabetic pain. Working closely with Dr
Hulse, you will gain high quality training in in vivo
and in vitro methodologies including rodent models of
diabetes, electrophysiological recordings and
measurement of microvascular blood flow in vivo,
experimental design and analysis and work within a
growing team of scientists passionate about
understanding disease processes in diabetes.
You should have a good
quality BSc in a relevant subject (neuroscience,
physiology, biomedical science, or equivalent).
To
enquire about the project and for more information on
applying, please email
Richard.Hulse@ntu.ac.uk


